Abstract
Background
PF-06838435 (SPK-9001), a gene therapy candidate containing a high specific-activity factor IX variant (R338L, FIX-Padua), is currently in phase 3 of clinical development for the treatment of Hemophilia B. Initial data with this vector is promising with significant reductions in bleeding episodes and FIX consumption (George LA et al, NEJM 2017; 377:2215-2217). To date, little is known about the activity of the expressed transgene product as measured in FIX:C one-stage and chromogenic assay systems commonly used to monitor FIX replacement therapy in patients with Hemophilia B.
Aim
The goal of the study was to assess the activity of the PF-06838435 expressed transgene product in plasma samples collected from participants in the Phase 1/2 trial using four commonly used FIX:C aPTT reagents. For comparison, the activity of the expressed FIX-Padua gene product was also assessed in the ROX FACTOR IX chromogenic assay. In addition, the activity of the Padua variant in congenital FIX deficient plasma spiked with increasing concentrations of purified recombinant human FIX Padua protein (rHFIXp - Samelson-Jones Lab, CHOP/UPenn), as well as recombinant human FIX (rHFIX, BeneFIX®, Pfizer Inc.), was assessed in the same FIX:C assay procedures.
Methods
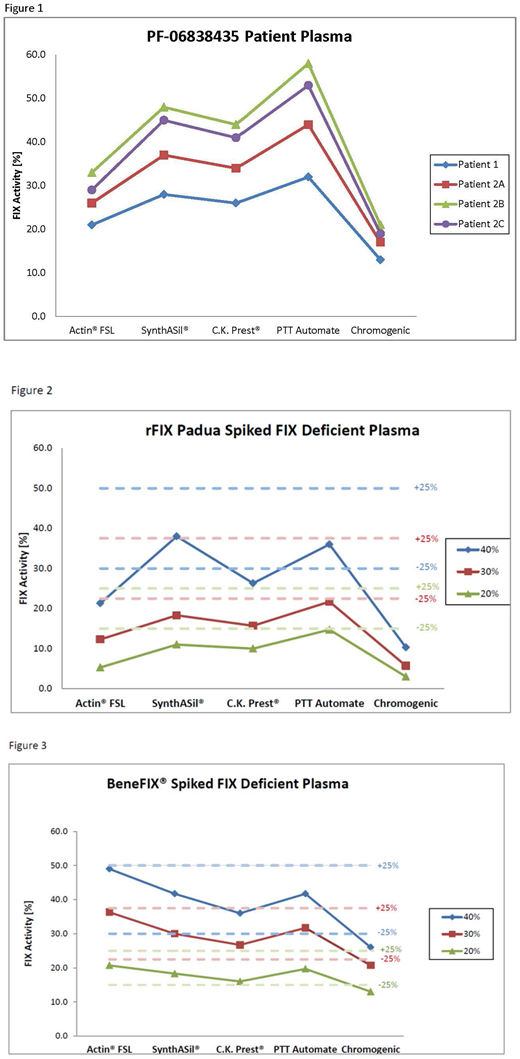
FIX:C, in four samples collected from two different patients who received FIX gene therapy, was tested in four in vitro diagnostic (IVD) approved FIX:C one-stage assay systems, STA®-PTT Automate and STA®-C.K. Prest® on the STA-R Evolution® (Diagnostica Stago Inc.), Dade Actin® FSL on the BCS®XP (Siemens Healthcare), and HemosIL® SynthASil® on the ACL TOP® (Instrumentation Laboratory). In addition, samples were also tested in the ROX FACTOR IX (Rossix AB) chromogenic assay on the BCS®XP (Siemens Healthcare). The aPTT reagents selected for this study correspond to 90% of the FIX:C assay reagents currently used in CAP accredited laboratories in the US(2014 CAP Proficiency Survey) and represent the three main types of activator commonly used in the FIX one-stage clot assay: silica (STA®-PTT Automate, HemosIL® SynthASil®), ellagic acid (Dade Actin® FSL) and kaolin (STA®-C.K. Prest®).
For comparison, FIX:C in each of the five FIX:C assay systems was also determined in samples spiked with purified rHFIXp (provided by Dr. Samelson-Jones) or rHFIX (provided by Spark Therapeutics Inc.) in 20X buffer solutions. On the day of testing rHFIXp, and rHFIX 20X buffer solutions were diluted 1:20 in congenital FIX deficient plasma to achieve approximate final FIX:C concentrations of 40, 30 and 20%, extrapolated from an estimated 8-fold specific activity to antigen ratio.
Results
A consistent pattern in the measured FIX:C for the PF-06838435 transgene product was observed in the five FIX:C assay systems (Fig. 1). For the aPTT based FIX:C assays, Actin FSL, gave the lowest FIX:C values, whereas PTT Automate measured the highest FIX:C levels. In all cases, the chromogenic FIX:C assay gave the lowest activity values for the transgene product. A similar FIX:C assay dependent pattern was observed for the purified rHFIXp spiked at 40, 30 and 20% FIX:C (Fig. 2). In all FIX:C assays tested, rHFIXp was under-recovered to a varying degree from target, with recoveries for the 20% FIX:C samples ranging between -26.5% (STA®-PTT Automate) and -73.5% (Dade Actin® FSL) in the aPTT based FIX:C assays and -85% in the ROX FIX chromogenic assay. In contrast, rHFIX (BeneFIX®) was recovered within ±25% of expected values in all aPTT based FIX:C assays and, consistent with previously reported data, modestly under-recovered in the ROX FIX chromogenic assay (Fig. 3).
Conclusion
This study found differences in the FIX:C results obtained for a Padua FIX variant transgene product and recombinant human FIX-Padua when tested in commonly used IVD approved FIX:C assays in North America. These results suggest that FIX:C assay selection is important for measuring FIX-Padua activity, which will be particularly relevant in hemophilia B gene therapy following FIX-Padua gene transfer.
George:University of Pennsylvania: Equity Ownership; Pfizer: Consultancy. High:Spark Therapeutics: Employment, Equity Ownership, Patents & Royalties. Carr:Sparks Therapeutics Inc.: Consultancy. Tiefenbacher:Laboratory Corporation of America: Employment, Equity Ownership; Siemens Healthcare: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal